mtrial
Congratulations to our DeepRAY project partners at Universitätsmedizin Mannheim for publishing a paper on „Streamlining Acute Abdominal Aortic Dissection Management“ based on an AI-based CT Imaging Workflow.
Within this InvestBW project funded by the Ministerium für Wirtschaft, Arbeit und Tourismus Baden-Württemberg, we will integrate the presented algorithm into mTRIAL and analyse the benefits of automatic AI-based image analysis on fast external servers for clinical routine and patient management.

Just imagine… evaluating brain perfusion on arterial spin labeling images was just one click away. Watch this video to see how we implemented Explore ASL in our mTRIAL platform to facilitate and standardize your next clinical study on neurodegenerative diseases.
Stay tuned to see how easy your data reaches mTRIAL.
Contact us for more information on ASL imaging and analysis.

Investigator-Initiated Trials (IITs) are pivotal in medical research, driven by independent investigators to explore new avenues and innovative medical approaches. These trials contribute significantly to advancing medical knowledge.
At mediri, we take pride in supporting researchers and clinicians in their IITs. With our expertise in imaging, we provide crucial assistance for these trials.
If you’re interested in learning more about our work or are involved in IITs, feel free to reach out to us.

Mediri is constantly evolving its image data management platform mTRIAL for secure and reliable central reads in clinical studies. Currently, mTRIAL is getting ready for RANO evaluation of low- and high-grade gliomas. Automatic interpolation of segmented volumes and easy annotation of measurements are on board. Stay posted.
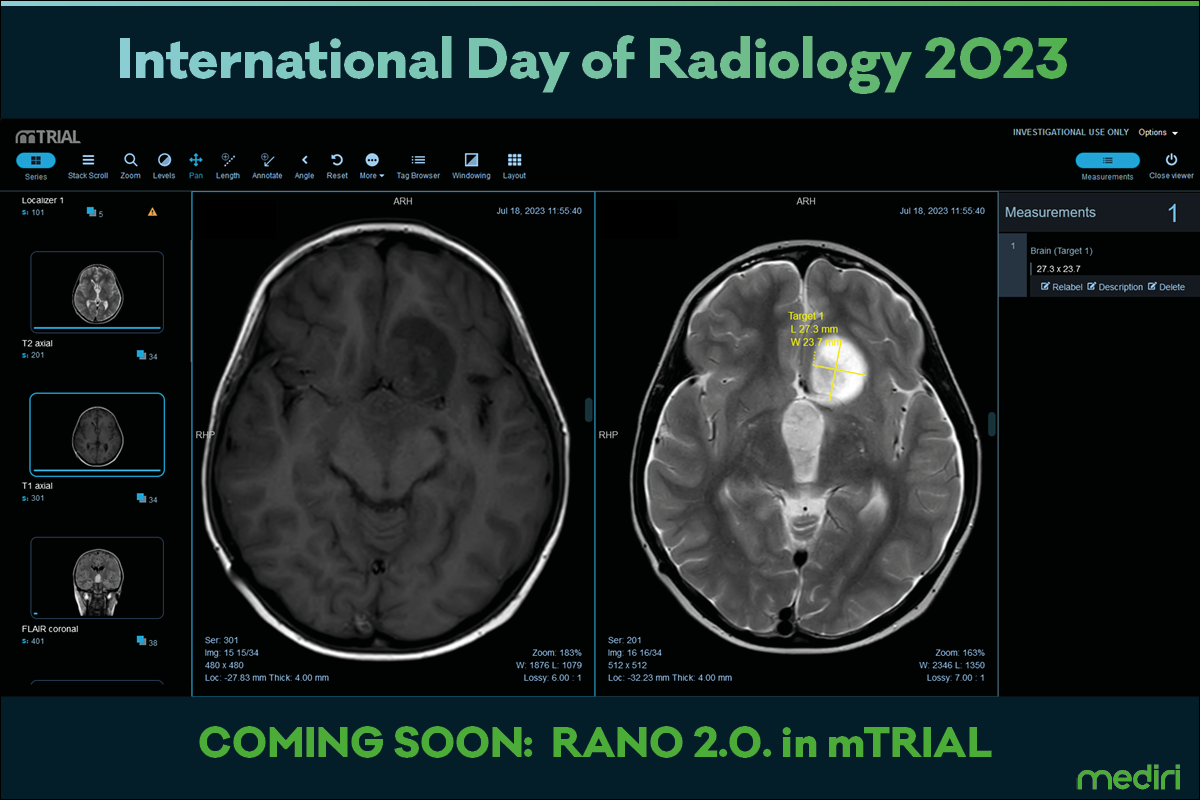
Today is International Day of Radiology to promote the seismic impact of radiology on medicine – earlier detection, fewer surgeries, and game-changing treatment outcomes. Analyzing radiological images in multi centric clinical studies can be challenging considering standardization and patient data privacy.
Mediri is constantly evolving its image data management platform mTRIAL for secure and reliable central reads in clinical studies. Currently, mTRIAL is getting ready for RANO 2.0 evaluation of low- and high-grade gliomas according to the recently published update by Wen et al. Stay posted.
We proudly announce the release of our new mTRIAL version. For the first time it incorporates a webbased comprehensive DICOM image viewer for platform independent and real time access of clinical trial data. Central read workflow planning and execution has been upgraded for even more reliable and efficient review of radiological data. mTRIAL 3.4. – ready to support your next image based clinical trial.

The easiest way to use FreeSurfer for brain volume measurements? Upload your FLAIR images to mTRIAL and enjoy the magic.
Interested? Don’t hesitate to contact us.
Our new mTRIAL release will come with a full featured browser based DICOM viewer on board. Designed for clinical trial data management, it supports segmentation, annotation and scoring tasks as defined in the study protocol. The OHIF based tool can be adapted to your personal needs and study requirements.
Curious? Contact us for a free demo via info@mediri.com.
We are excited to announce that mediri is working together with the Universitätsmedizin Mannheim on the project “DeepRAY“. The goal of the project is to extend mediri’s mTRIAL platform for AI-based detection of aneurysms and dissections of the aorta on CT images. This will improve the prognosis of patients with life-threatening changes in the abdominal aorta. The investBW project will run for two years and is funded by the Ministerium für Wirtschaft, Arbeit und Tourismus Baden-Württemberg.

We are delighted to share that we have a new Chief Operations Officer: Julia Müller will from now on take care of mediri’s fortune. She takes over from Prof. Johannes Gregori who will be remaining on board as mediri’s Director of Research.
