stuttgart
Recently, a newspaper article about our project with bosomshield got mentioned in the newspaper echo-online. If you want to know more about the importance of our work and how we want to improve the field of breast cancer, you can read the article down below.

Today is International Day of Radiology to promote the seismic impact of radiology on medicine – earlier detection, fewer surgeries, and game-changing treatment outcomes. Analyzing radiological images in multi centric clinical studies can be challenging considering standardization and patient data privacy.
Mediri is constantly evolving its image data management platform mTRIAL for secure and reliable central reads in clinical studies. Currently, mTRIAL is getting ready for RANO 2.0 evaluation of low- and high-grade gliomas according to the recently published update by Wen et al. Stay posted.

New office. New Neighbours. New view.
It’s done! We have offically moved to Am Taubenfeld 21/1 in 69123 Heidelberg.

These numbers again stress the importance of research in breast cancer detection and relapse prediction. We are proud to be part of the Marie-Sklodowska Curie network bosomshield.eu aiming at the combination of clinical, pathological and radiological data for better treatment and therapies. Still, early detection of breast cancer is one of the most important factors for successfull treatment, so be mindful and take care of yourself!

We proudly announce that mediri has been awarded as Digital Innovator 2023 in the category „medical technology and pharmaceuticals“ by CHIP. In times of digitization, CHIP and data analysis specialist Globis Consulting have studied the innovative power of German companies and institutes in digitization. CHIP is known as one oft the top recommendation brands.
See the full ranking here.

You want the brain MRIs of your MS patients to be evaluated by the SPM Lesion Segmentation Tool but without installing and adapting any software packages? Or do you need consistent evaluations across multiple centres of a clinical trial? mTRIAL offers Lesion analysis of FLAIR images based on the SPM LST algorithm and produces clear reports. You only need to upload your image data to the mTRIAL platform, pseudonymization is performed on the fly, evaluation and reporting can be done within minutes.
Interested? Don’t hesitate to contact us.
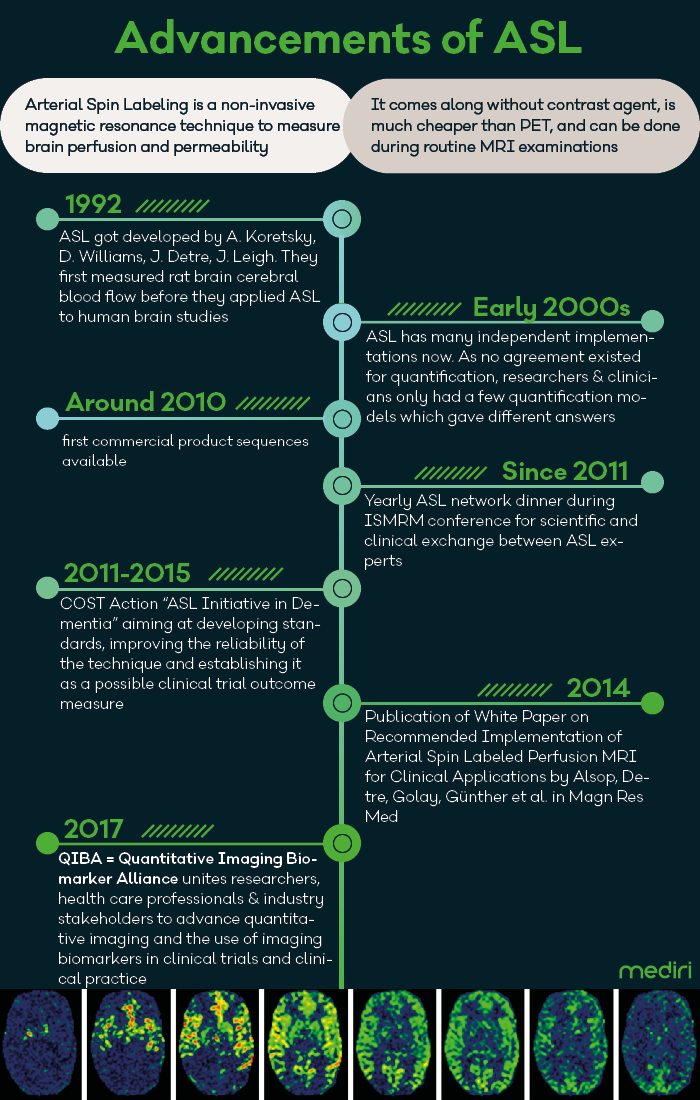
30 years after the first successful Arterial Spin Labeling (ASL) MR image acquisition to measure cerebral blood flow in rat brain, the early detection of Alzheimer’s using ASL gains enormous importance with the recent publication of the study results for Lecanemab. Now that there is hope for efficient therapies in dementia diseases, it is a good time to review the steps that have been gone since then. Many initiatives aiming at reliable and comparable quantification of brain perfusion measurements are paving the way for broad usage of ASL in clinical studies focusing on Alzheimer‘s and other dementia diseases.
We are looking forward to the installation of our WHITEBOX at the Universitätsmedizin Mannheim. It will allow for fast and pseudonymized DICOM image transfer from CT scanner to mTRIAL study server. The results of AI based image analysis will be sent directly to the radiologist on duty.
Also looking for easy DICOM upload options? Contact us via info@mediri.com.

In honor of todays International Day of Radiology, we want to thank again the attendees of the 8th International Symposium on Focused Ultrasound (23.10.2022 – 27.10.2022) for valuating the contribution of our colleague Jürgen Jenne. The topic covered the first steps towards an automated and generalized definition of target and areas of risk in tcMRgFUS (transcranial therapy with MR-guided focused ultrasound) for tremor therapy. One part of the presented work was to establish a robust method for fiber tracking on brain MR images.

Our new mTRIAL release will come with a full featured browser based DICOM viewer on board. Designed for clinical trial data management, it supports segmentation, annotation and scoring tasks as defined in the study protocol. The OHIF based tool can be adapted to your personal needs and study requirements.
Curious? Contact us for a free demo via info@mediri.com.