News
Unidirectional feature for RECIST MRI
16. October 2025
Next up in our mTRIAL release preview:
the Unidirectional Feature for RECIST MRI — a key advancement in longitudinal lesion tracking and precision oncology.
Users can now measure lesion diameters with pinpoint accuracy, activate a second viewing window to compare follow-up exams, and adjust views to assess additional lesions — all within one streamlined workflow. Seamless transitions between examinations make longitudinal analysis faster and more intuitive than ever.
Breast Cancer Awareness Month
08. October 2025
October marks Breast Cancer Awareness Month — a global initiative to promote prevention, early detection, and innovative research against breast cancer.
We’re proud to contribute to this mission through our involvement in the Marie-Skłodowska-Curie network BosomShield. Together, we’re advancing breast cancer treatment by integrating clinical, pathological, and radiological data.
Our PhD researchers are developing AI-driven methods for lesion segmentation and classification across multiple imaging modalities — aiming to improve relapse probability prediction and support more personalized care.
Let’s keep pushing boundaries in research and raising awareness.

Sneak peek: Spline Feature for RECIST CT
30. September 2025
Next up in our mTRIAL release preview: the Spline Feature for RECIST CT — a game-changer in lesion annotation and precision oncology.
Users can now outline target lesions point by point, creating smooth, adaptive contours for complex shapes. With additional viewing windows, it’s easy to compare multiple studies side by side — unlocking deeper clinical insights.
More innovations coming soon!
Sneak peek: The Tridirectional Feature
25. September 2025
This week, we introduce the Tridirectional Feature — empowering users to angulate in three dimensions and measure tumor size across three orthogonal directions with precision and ease.
A powerful step forward in volumetric analysis and clinical insight.
Stay tuned — more innovations are on the horizon!
World Alzheimer’s Day
22. September 2025
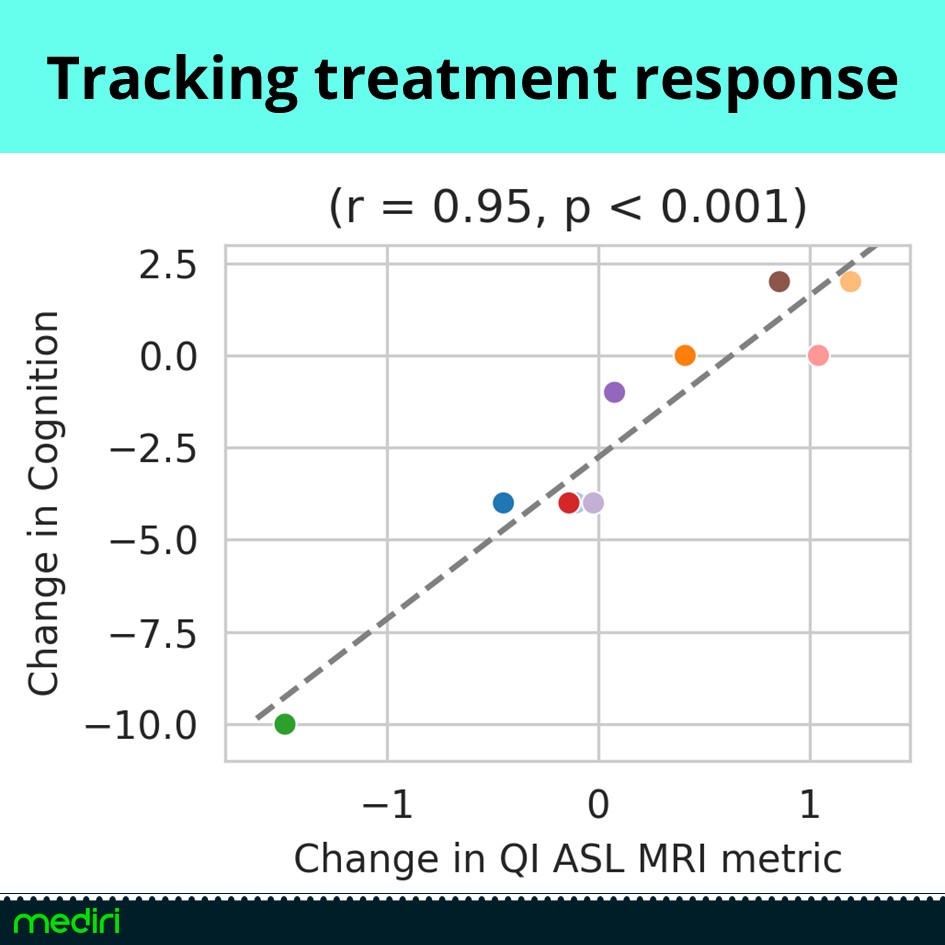
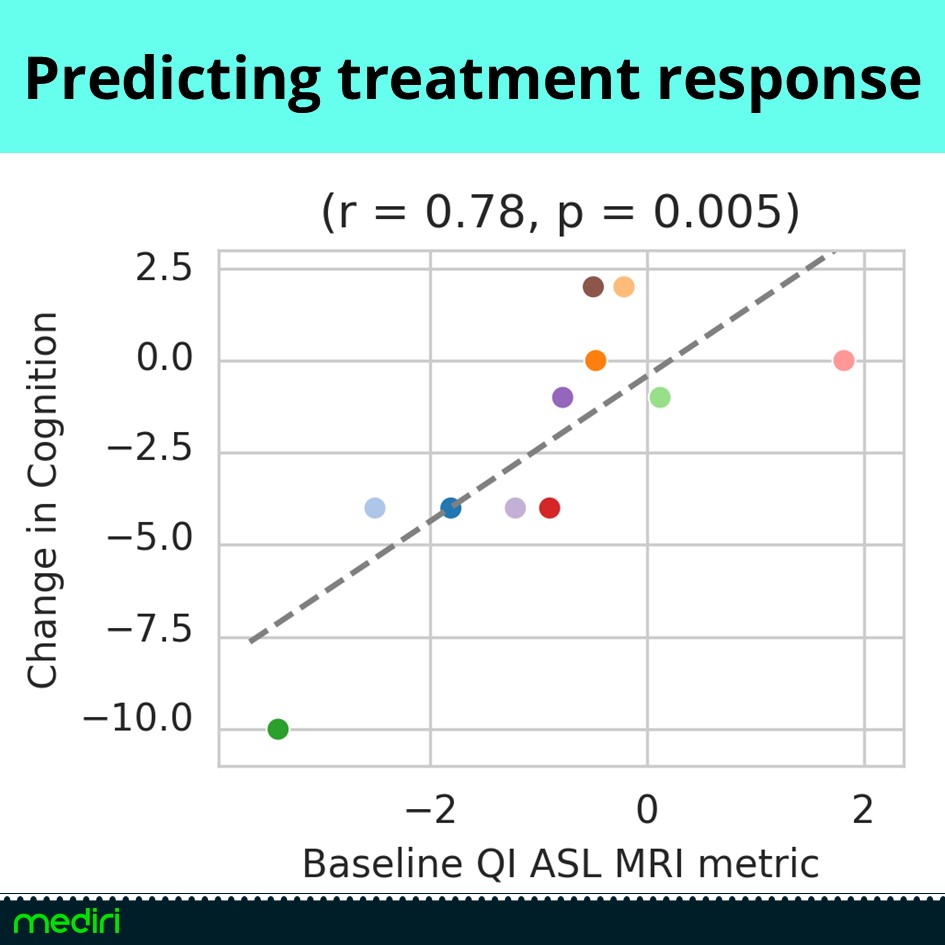
Hot of the press results from out partner Quantified Imaging prove the potential of ASL MRI for dementia research.
We offer powerful imaging methods and analysis to support treatment response evaluation in clinical trials for Alzheimer’s disease.
– ASL MRI shows strong correlation between perfusion change and cognitive improvement under Lecanemab therapy — outperforming volume-based metrics.
– Baseline prediction highlights ASL’s potential to identify responders before treatment begins.
A non-invasive, cost-effective alternative to PET — ideal for clinical trials and routine care.

Sneak peek: The Freehand Feature
17. September 2025
Next Sneak Peek: The Freehand Feature.
Continuing our journey through the new mTRIAL release!
After showcasing the precision of the Spline tool, we now present the Freehand Feature — designed for intuitive and flexible lesion outlining.
More exciting features are just around the corner — stay connected!
Sneak peek: The Spline Feature
09. September 2025
Third Sneak Peek: The Spline Feature
Another exciting reveal from the upcoming mTRIAL release!
Following the Lifewire tool, we’re thrilled to introduce Spline — a precision-driven feature that allows users to draw lesion outlines point by point. This manual approach gives full control over the shape and detail of the region of interest, making it ideal for complex or irregular lesions that require expert-level accuracy.
Whether you’re refining segmentation or preparing data for analysis, Spline empowers you to work with confidence and clarity.
Stay tuned — more innovations are on the way to transform your imaging workflow!
Sneak Peek: the Livewire Feature
04. September 2025
We’re back with another exciting feature from our upcoming mTRIAL release.
Second Sneak Peek: The Livewire Feature
This powerful tool enables users to draw lesion outlines with threshold-based segmentation support, making it easier than ever to define and analyze regions of interest in medical imaging.
This is just one of many innovations coming your way — stay tuned for more features that will elevate your imaging experience!
Coming Soon: A new mTRIAL Release
19. August 2025
The next version of mTRIAL is just around the corner — and it’s bringing a powerful upgrade to our DICOM Viewer!
First Sneak Peek: The Angulation Feature
With this new tool, you can:
- Angulate in three dimensions for precise anatomical alignment
- Measure in the selected imaging plane to enhance diagnostic accuracy
Stay tuned — more innovative features are on the way!
Team Outing
12. August 2025
Last Friday, our Heidelberg department swapped desks for hiking boots and ventured into the beautiful forests of the Palatinate region for a team outing.
We followed a scenic circular trail to the Hohe Loog Haus, passing the Nollenkopf and several breathtaking viewpoints along the way. Shaded by towering trees and surrounded by nature, the 10 km route offered the perfect setting to reconnect and recharge.
At the hut, we relaxed under chestnut trees and enjoyed delicious regional specialties — Esse & Trinke at its finest!
And the cherry on top: our COO, Julia Müller invited us to her home afterwards, where she treated us to her legendary homemade nut braid (Nusszopf) — a true highlight and a heartfelt gesture that made the day even more special.
A big thank you to everyone for this great day.







