
Our software solution for clinical studies
mTRIAL is based on the practical challenges of multicentre clinical trials. The software solution is individually configurable, scalable and adapts to your requirements. Optional modules range from image data uploads with embedded pseudonymisation and automatic quality checks to personalised reporting and monitoring tools – irrespective of the clinical indication and the chosen imaging modality.
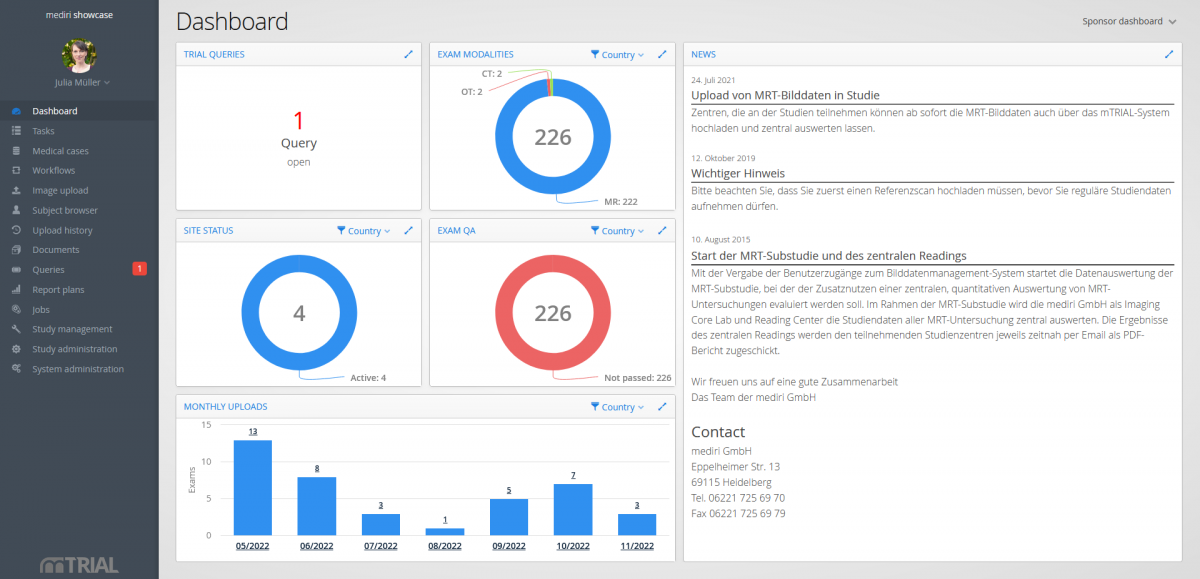
Keep track…
… of your study progress in real-time via role-specific dashboard.
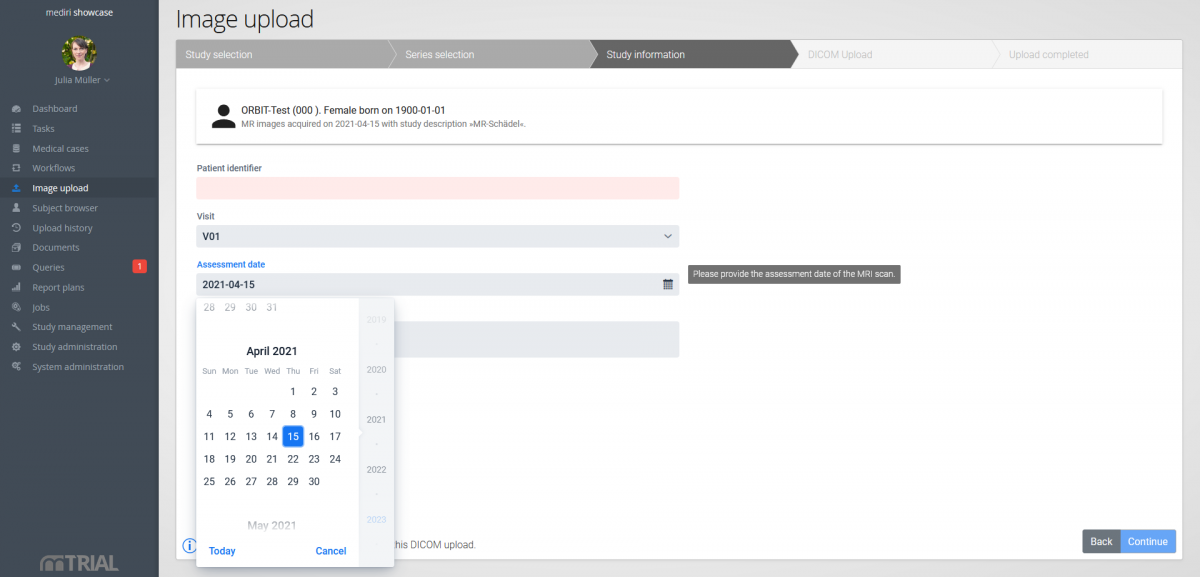
Image data…
… is automatically pseudonymised before upload. Additional study information can be captured on the web surface according to your specifications.

- mTRIAL has a full audit trail and is compliant with applicable regulations (ICH E6, ICH E8, 21 CFR Part 11, HIPAA)
- Administration of trial sites and users (site staff, readers, sponsor) is intuitive and provides adjustable roles and access rights.
- Our service is scalable and highly customized to the specific requirements of a study, no matter which indication is in focus.
- The imaging review charter is translated into workflows and user tasks for highest compliance to the study protocol.
- mTRIAL features real-time feedback to sites and sponsors and covers the complete query process for image data.
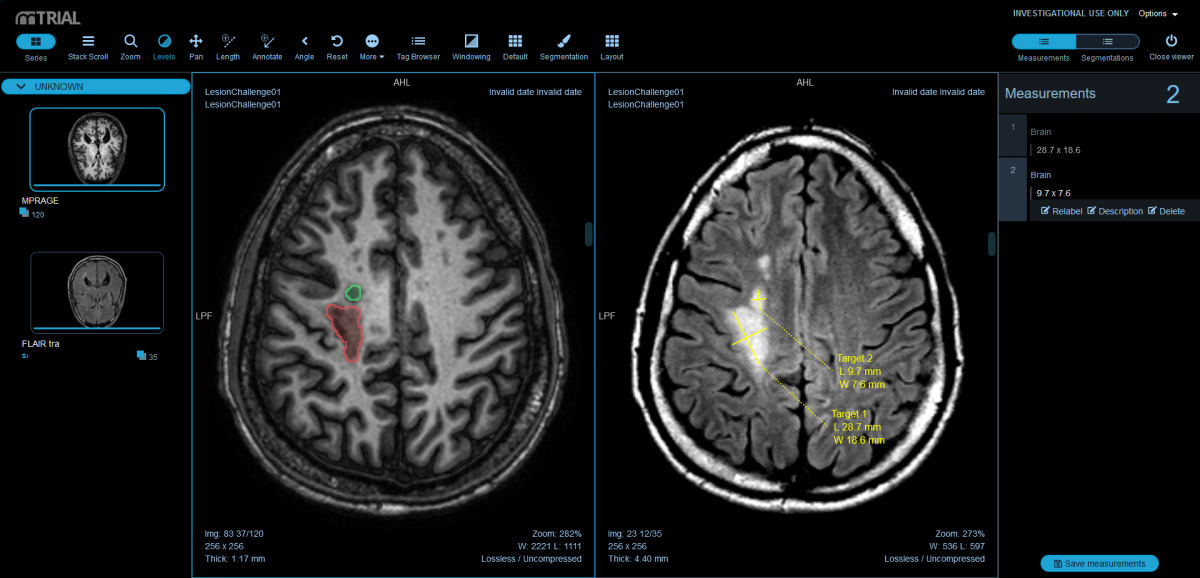
DICOM-viewer…
… access your medical image data from anywhere via web-based DICOM viewer – for more efficient workflows and reliable results.
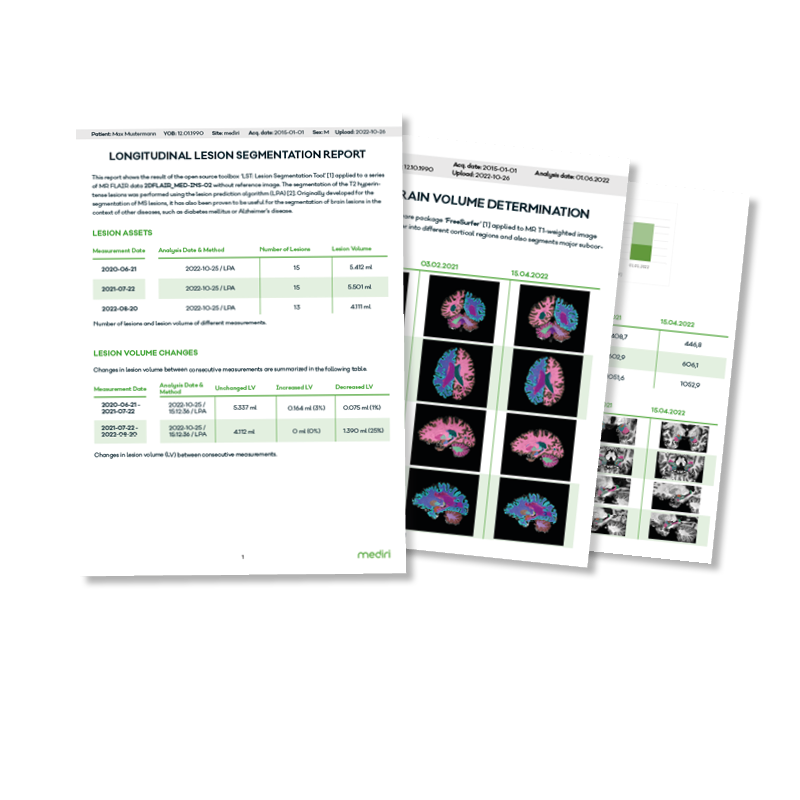
Always up to date…
… stay up to date with automatically generated system and case reports, which can also be distributed via eMail.
Data protection & data security
guarantees SSL-encrypted
data transmissions
hosted in
German data centres
exclusively processes
pseudonymised data
developed according to the
EN ISO 13485 standard